Fundamental new mechanism in neuroscience
NoNO’s core science is published, well-established and reproducible. Initially discovered by NoNO, PSD-95 inhibition has now been validated both internally and externally by numerous independent research groups worldwide with preclinical results in stroke, stroke recovery, Alzheimer’s, anxiety, epilepsy, retinal ischemia and traumatic brain injury. Our core technology is wholly owned and supported by a portfolio of more than 300 granted patents globally.
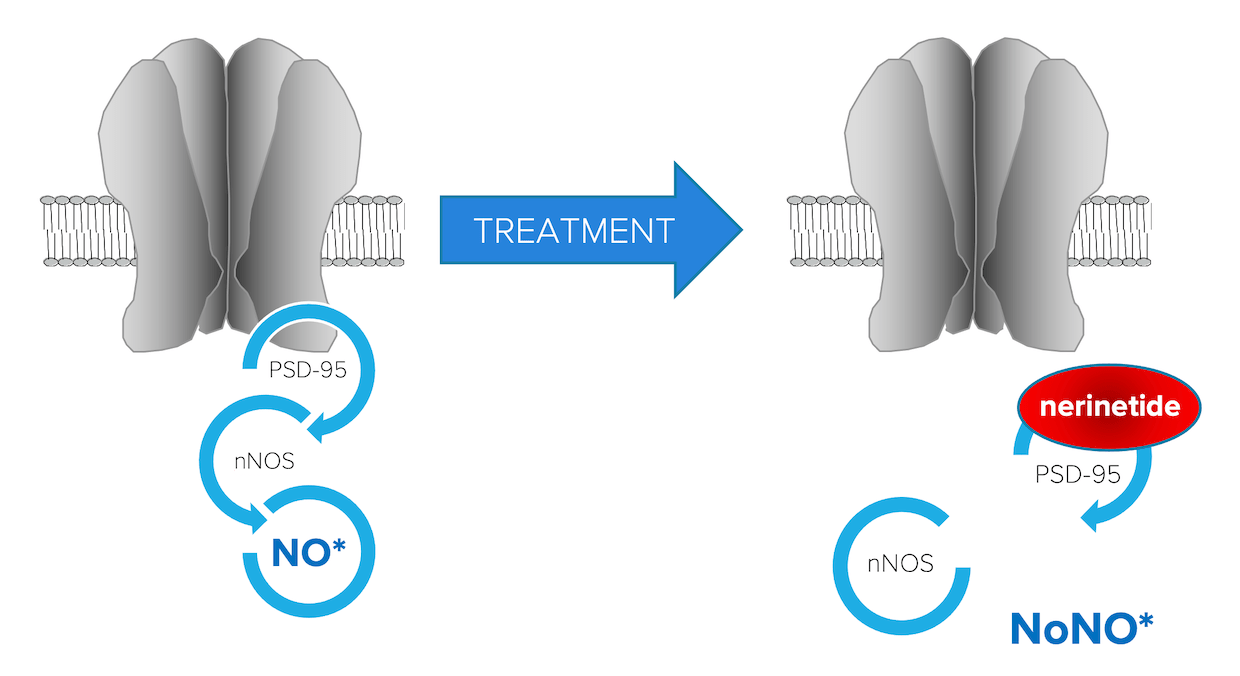
NoNO’s pipeline is built around its lead asset nerinetide, a first-in-class peptide inhibitor of PSD-95 (postsynaptic density protein 95) which crosses the blood brain barrier and binds PSD-95 and the enzyme neuronal nitric oxide synthase (nNOS) to mediate its effects. PSD-95 protein is the essential linker between NMDA glutamate receptors and nNOS responsible for generating the damaging reactive species nitric oxide following both ischemic and mechanical stretch injuries. Nerinetide and its analogs disconnect this link, protecting neurons in the acute injury phase and promoting neural plasticity for functional restoration in the recovery phase.
Multiple Opportunities for therapeutic intervention
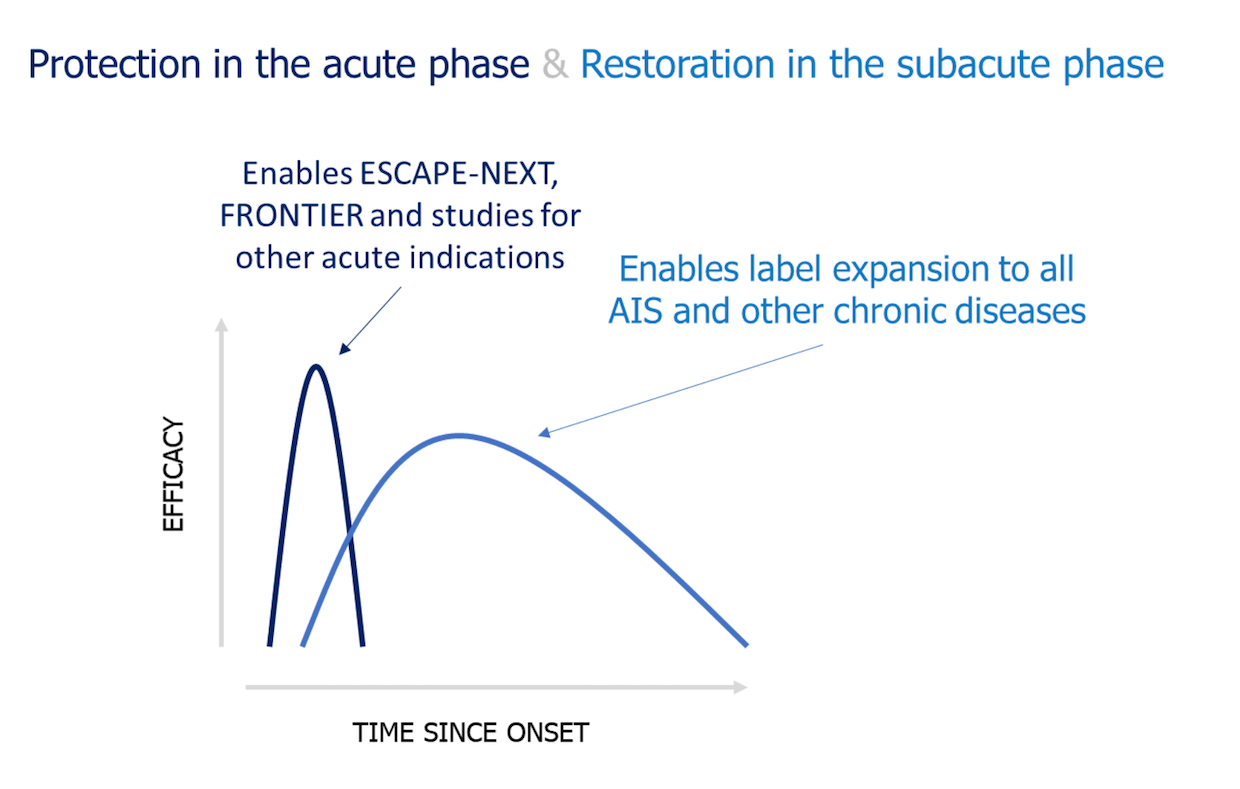
This fundamental mechanism of action by nerinetide and its analogs – dampening excitotoxicity and enhancing neural plasticity – enables multiple opportunities for therapeutic intervention across a broad range of CNS indications. In the acute phase, indications as diverse as acute ischemic stroke (AIS), subarachnoid hemorrhage (SAH) and traumatic brain injury (TBI) share a key therapeutic opportunity – excitotoxic signalling through PSD-95 and nNOS that threatens brain tissue which survived the initial injury but remains at risk. Nerinetide and its analogs have been developed to protect such patients with ‘brain left to save’. In the chronic phase, nerinetide enhances neuroplasticity and functional recovery in preclinical models of AIS even when treatment does not begin until days after the acute phase of brain cell death is complete. Therefore, nerinetide may offer opportunities for a better life not just for people experiencing an emergent stroke but also for many of the 101 million stroke survivors who remain on the road to recovery.
PUBLISHED, ESTABLISHED & REPRODUCIBLE
KEY PHARMACODYNAMIC REFERENCES
- Sattler R, XiongZ, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845-1848
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846-850
- Cui H, Hayashi A, Sun H, Belmares MP, Cobey C, Phan T, et al. PDZ protein interactions underlying NMDA-receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. Journal of Neuroscience. 2007;29:9901-9915
- Cook DJ, Teves L, Tymianski M. A translational paradigm for the preclinical evaluation of the stroke neuroprotectant Tat-NR2b9c in gyrencephalic nonhuman primates. Sci TranslMed. 2012;4:154ra133
- Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012 Feb 29;483(7388):213-7.
- Mayor-Nunez D, Ji Z, Sun X, Teves L, Garman JD, Tymianski M. Plasmin-resistant PSD-95 inhibitors resolve effect-modifying drug-drug interactions between alteplase and nerinetide in acute stroke. Sci Transl Med. 2021 Apr 7;13(588):eabb1498.
